– Updated on August 18, 2025
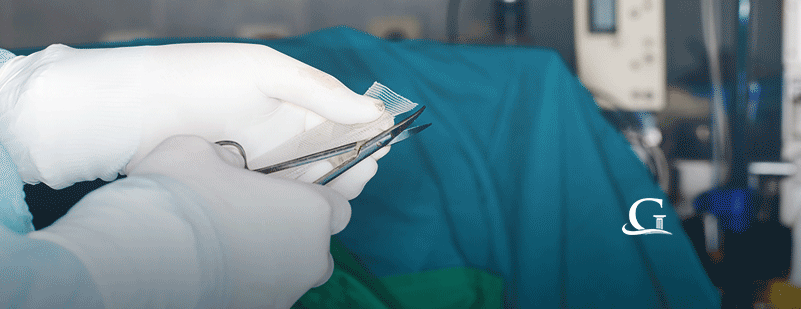
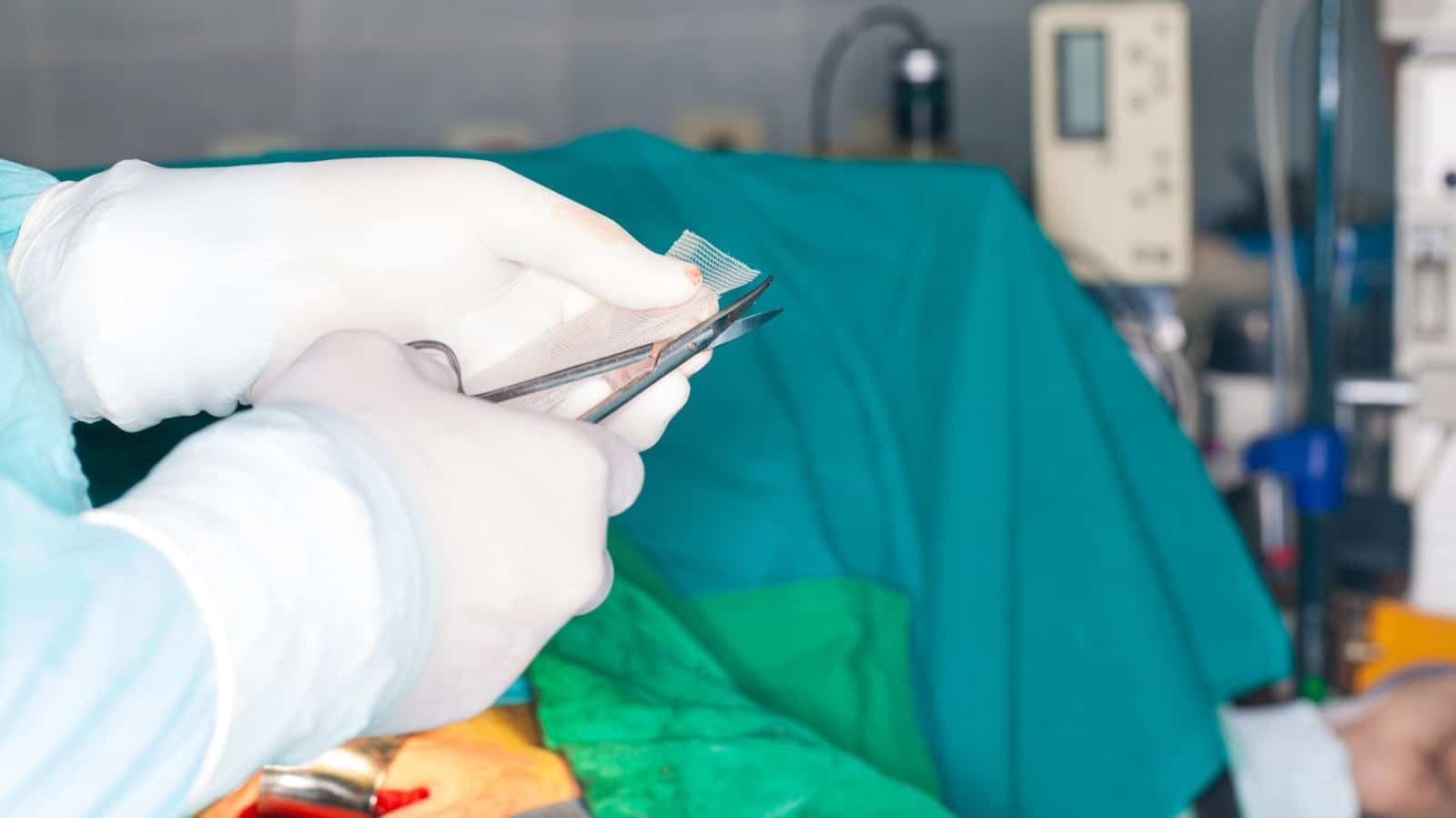
Hernia mesh lawsuits filed against Atrium, manufacturer of C-Qur mesh, continue. Recently, one plaintiff alleged that he suffered more than an injury from the mesh. James Brown, who was a recipient of C-Qur mesh to correct an umbilical hernia, also alleged that the materials used to manufacture it are biologically incompatible with human tissue.
What Is C-Qur Mesh Made From?
C-Qur mesh is comprised of polypropylene and coated in omega-3 fatty acid fish oil. According to the brochure for C-Qur mesh, the purpose of the omega-3 fatty acid fish oil coating is to minimize the risk of the surrounding tissue becoming attached to it.
Is C-Qur Mesh Biologically Incompatible?
Brown’s hernia mesh lawsuit alleges that the polypropylene used to create C-Qur mesh is biologically incompatible. After Brown’s umbilical hernia was repaired using C-Qur mesh, he continued to experience excruciating pain. Ultimately, the C-Qur mesh was removed. A pathology report associated with the removal stated that the C-Qur mesh caused a “foreign body giant cell reaction.” The reaction wasn’t just limited to the hernia repair. Scar tissue developed and was also affected.
Brown’s lawyer also alleged that the packaging design for the C-Qur mesh did not have enough moisture. The purpose of moisture is two-fold. C-Qur mesh is treated with a sterilizing agent in order to reduce the likelihood of bacteria developing. The lack of moisture means that the effectiveness of the sterilizing agent is reduced. It also prohibits the omega-3 fatty acid fish oil from properly adhering to the C-Qur mesh.
Schedule A Free Case Review!
What About The Omega-3 Fatty Acid Fish Oil?
Atrium boasts in their brochure that their C-Qur mesh is coated with omega-3 fatty acid fish oil to discourage the surrounding tissue from attaching itself to the mesh. However, many people filing hernia mesh lawsuits experienced just that. Additionally, Brown’s hernia mesh lawsuit noted an additional risk involving the fish oil. It’s not something that’s often mentioned. This makes C-Qur mesh a legitimate life or death risk to anyone with a fish allergy. Brown alleged that Atrium knew it could affect people, but did what they could to keep the potential problem quiet.
Additionally, the FDA was not notified by Atrium about their change in the application of the omega-3 fatty acid fish oil. This amounts to a change in the design of a medical product.
Did Atrium Fail To Investigate?
Another important allegation of Brown’s hernia mesh lawsuit is that Atrium failed to investigate reports of adverse reactions, failure to report those adverse reactions to the FDA, and although they halted production, they did not notify medical professionals or the FDA. Ultimately, the court will determine whether Atrium failed to properly investigate the reports that they received regarding C-Qur mesh.
Would You Benefit From A Hernia Mesh Lawsuit?
If you underwent surgery to correct a hernia and were made sick or injured because of C-Qur mesh, you may benefit from a hernia mesh lawsuit. You may be entitled to compensation for your medical care, pain, and suffering. Goldwater Law Firm provides free case evaluations. To schedule your free case evaluation, contact us today.

The Goldwater Law Firm is on mission to help as many people as possible with the fierce, compassionate legal aid only The Gold Standard of Injury Law can offer. If you suffered serious side effects or were diagnosed with an illness because of a defective drug or product, or if you were injured in an accident that wasn’t your fault, Attorney Bob Goldwater and the Goldwater Law Firm is ready to serve as your compassionate partner in the fight to seek the compensation and justice you deserve.
Share this post: