Table of Contents
According to the manufacturers, over 1 million people have used Dupixent to treat allergic conditions and other illnesses. While dupilumab, Dupixent’s generic name, can be effective in managing these diseases, recent studies have linked its use to an increased risk of cancer.
If you used Dupixent and then received a cancer diagnosis, you may be eligible to file a Dupixent T-cell lymphoma lawsuit. Our team details the lawsuit and how a Dupixent CTCL skin cancer lawyer can help you below.
Getting Help is Easy
Call Us Anytime. Nights And Weekends, We’re Available.
A Trusted, Experienced Partner
Fill out the form on the top of the page and a case specialist will reach out to you.
No Fees Unless We Win
No Win, No Fee. You Don’t Pay A Penny Unless We Win Your Case.
Can I Afford a Lawyer for My Dupixent T-Cell Lymphoma Lawsuit?
Yes. The Goldwater Law Firm partners with co-counsels nationwide who handle cases on a contingency basis. This ensures you can access a law firm near you that will take your case with no upfront fees. You only pay your attorney if and when they win your case.
What Illnesses Are Associated With Dupixent Use?
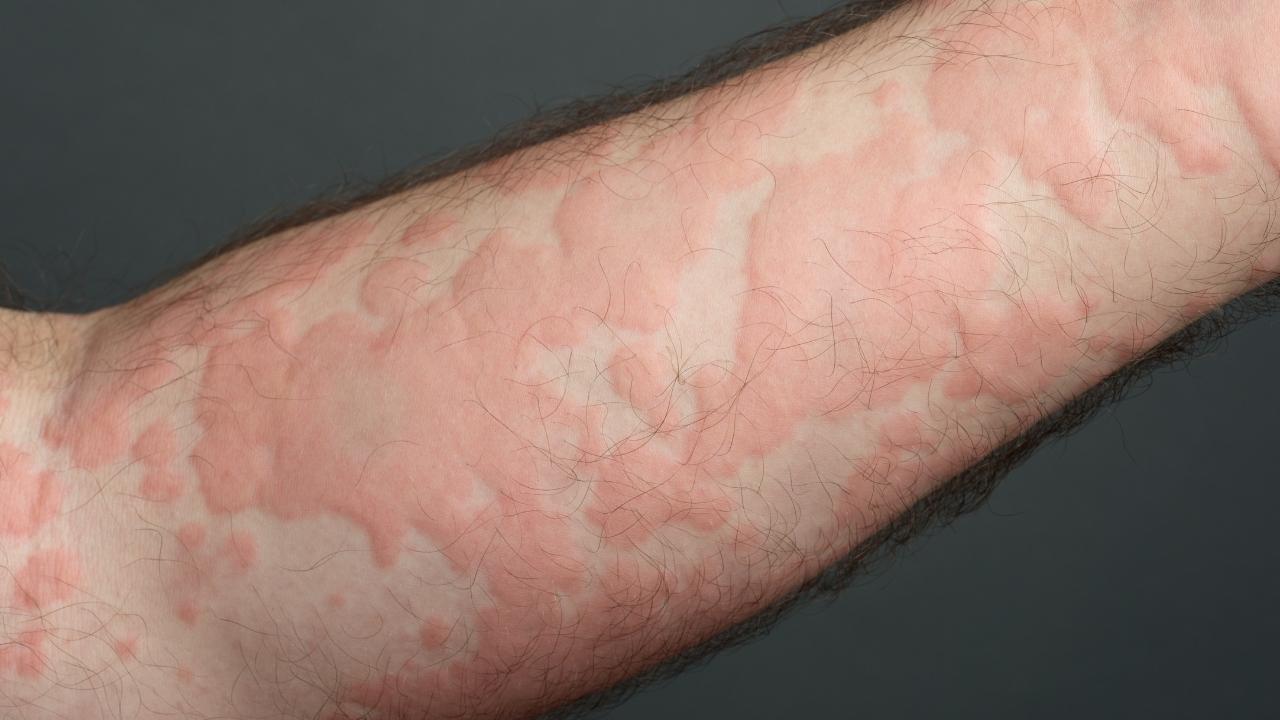
Several studies have linked dupilumab use with an increased risk of cutaneous T-cell lymphoma (CTCL).
A 2024 study published in the Journal of the American Academy of Dermatology found an increased risk of cutaneous T-cell lymphoma among people with atopic dermatitis (eczema) who used dupilumab. The study found a 300% increase in the risk of developing CTCL in dupilumab patients.
Another 2024 study published in Dermatologic Therapy also found an increased risk of CTCL in people who used dupilumab to treat their atopic dermatitis.
There are over a dozen subtypes of cutaneous T cell lymphoma, including:
- Mycosis fungoides (MF)
- Mycosis fungoides variants
- Folliculotropic MF (FMF)
- Pagetoid reticulosis (PR)
- Granulomatous slack skin (GSS)/granulomatous mycosis fungoides
- Sézary syndrome
- Subcutaneous panniculitis-like T-cell lymphoma (SPTCL)
- Primary cutaneous CD30+ lymphoproliferative disorders
- Lymphomatoid papulosis (LyP)
- Primary cutaneous anaplastic large cell lymphoma (C-ALCL)
- Primary cutaneous peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS)
- Primary cutaneous gamma delta T-cell lymphoma (PCGD-TCL)
- Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma
- Primary cutaneous acral CD8+ T-cell lymphoma
- Primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder
Thinking About Filing a Dupixent Skin Cancer Lawsuit?
What Is the Dupixent Skin Cancer Lawsuit?
Plaintiffs have filed lawsuits against the makers of Dupixent, claiming that the drug increases the risk for CTCL and that the makers failed to adequately warn doctors and patients.
While many of the studies are newer, Sanofi Genzyme and Regeneron Pharmaceuticals have had at least a year to look into patient reports and studies and warn those prescribing and using their product.
Dupixent Updates
The Dupixent lawsuits are still in the very early stages. We will continue to update you when we receive more information about the lawsuits that have been filed.
September 2024
The U.S. Food and Drug Administration (FDA) approved Dupixent to treat COPD.
August 2024
A study published in Dermatologic Therapy found that atopic dermatitis patients treated with dupilumab have a higher risk of developing cutaneous T-cell lymphoma than those who never received a dupilumab dose. According to the study, “there were no CTCL cases in patients younger than 60 in the group not receiving dupilumab.”
August 2024
A study published in the Journal of the American Academy of Dermatology found an increased risk of cutaneous T-cell lymphoma in people who treated their atopic dermatitis with dupilumab. In fact, the study found a 300% increase in the risk of developing CTCL.
Book Your Free Case Review
September 2022
The FDA approved Dupixent for the treatment of prurigo nodularis.
May 2022
The FDA approved Dupixent for the treatment of eosinophilic esophagitis.
June 2019
A study in the European Respiratory Journal found that people prescribed dupilumab for their asthma had a higher risk of developing lymphomas.
June 2019
The FDA approved Dupixent for the treatment of chronic rhinosinusitis with nasal polyps.
October 2018
The FDA approved Dupixent for moderate-to-severe asthma.
March 2017
The FDA approved Dupixent for the treatment of atopic dermatitis.
Frequently Asked Questions
You may be eligible to file a lawsuit if you:
- Used Dupixent for your atopic dermatitis (eczema), asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, prurigo nodularis, or chronic obstructive pulmonary disease (COPD)
- Were diagnosed with CTCL or a subtype at least a month after your first Dupixent dose
You will likely also need to prove that a doctor did not diagnose you with CTCL, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, non-follicular lymphoma, leukemia, or skin cancer before you were prescribed Dupixent.
Our team and co-counsels can help you determine whether you may qualify.
Being diagnosed with cutaneous T-cell lymphoma after taking Dupixent can be overwhelming, medically, emotionally, and financially. A lawsuit may allow you to pursue compensation for the wide range of losses caused by this diagnosis. These damages are not limited to just medical bills; they can also include the income you’ve lost, the suffering you’ve endured, and how your life and family relationships have been affected. Below are the main categories of recovery that plaintiffs in Dupixent CTCL cases may seek.
Medical Expenses
You may be able to recover costs such as:
- Diagnostics, e.g., skin biopsies, imaging, and pathology lab work
- Treatments, e.g., topical therapies, systemic treatments, and radiation
- Hospitalization and surgeries, if required
- Ongoing care, such as follow-ups, medications, specialist visits, and home care
Lost Wages and Earning Capacity
Recovery may include:
- Income lost during treatment or recovery periods
- Reduced ability to work long-term if CTCL causes lasting impairments
- Loss of promotions or career opportunities due to the inability to perform prior job tasks
Pain and Suffering and Mental Anguish
You may recover for:
- Physical pain from CTCL itself and treatments
- Emotional distress, e.g., anxiety, depression, and fear of death
- Loss of self-esteem, disfigurement, and changes in body image
Loss of Enjoyment of Life
If CTCL or its treatment prevents you from enjoying hobbies and family events or otherwise diminishes quality of life, that may be compensable.
Pain and Emotional Distress for Family or Loss of Consortium
If a serious illness (or wrongful death) impacts close family members, some states allow recovery for their emotional losses or loss of companionship.
Attorneys’ Fees and Costs
Most product liability/failure-to-warn cases allow recovery of your attorneys’ fees and the cost of building your case if your attorney wins.
Other Related Costs
Other recoverable items might include:
- Travel to and from medical appointments
- Lodging if treatment is far from home
- Medical equipment and modifications to the home, if needed
- Caregiving expenses
As of September 2025, there are no publicly disclosed settlements or verdicts for Dupixent cases. We will update with any lawsuits or settlements as they become available.
No, as of September 2025, the Dupixent lawsuit is not a class action. Plaintiffs have filed lawsuits individually. The plaintiffs and their attorneys may request that a court classify the cases as a class action or multidistrict litigation in the future.
The Dupixent lawsuits are in the earliest stages. Plaintiffs have filed lawsuits, but there are no settlements or verdicts to speak of, and none of the cases have gone to court.
Research is also in the early stages, but as more information becomes available, we can expect more plaintiffs to file claims and lawsuits.
It’s natural to wonder what your Dupixent case may be worth, but there’s no way to know without a thorough investigation into your situation. Every claim is unique, and the potential value depends on many factors, including:
- Your specific injuries and medical complications
- The severity of your cancer
- How long it will affect you
- The cost of past and future medical care
- Lost wages and loss of earning capacity
- Pain and suffering, emotional distress, and reduced quality of life
- Other damages tied directly to Dupixent’s impact on your life
Because no Dupixent lawsuits have reached a settlement or jury verdict, there is no historical data to help predict what juries might award or what defendants may offer to settle cases. Until settlements are negotiated or trial outcomes are known, any estimate would be speculative.
The value of a Dupixent case can only be determined after gathering evidence, documenting your full damages, and understanding how the litigation develops. If you believe you’ve been harmed by Dupixent, the best step is to speak with an attorney who can review your circumstances and help you pursue fair compensation.
If you believe Dupixent may have contributed to your skin cancer diagnosis, a lawyer can play a vital role in protecting your rights and guiding you through the legal process. An experienced attorney will start by carefully reviewing your medical history, prescription records, pathology reports, and timeline of Dupixent use to determine whether you may have a viable claim. They can also coordinate with oncologists, dermatologists, and other medical experts to evaluate the connection between your diagnosis and the drug, ensuring your case is supported by credible evidence.
Investigating potential corporate negligence is another key step. Your lawyer can examine whether the manufacturer failed to test Dupixent adequately, withheld information about known risks, or failed to provide clear warnings to patients and physicians. By uncovering internal documents, clinical trial data, and regulatory filings, your attorney can build a strong case showing how the drug company may have prioritized profits over patient safety.
Beyond building the foundation of your claim, a lawyer shields you from the pressure tactics of pharmaceutical companies and insurance providers. They can take over all communications, file the necessary court documents, and negotiate for fair compensation on your behalf. If a reasonable settlement cannot be reached, your attorney is prepared to present your case in front of a jury.
Plaintiffs have filed lawsuits against Sanofi Genzyme and Regeneron Pharmaceuticals.
While the manufacturers of Dupixent, Sanofi Genzyme, and Regeneron Pharmaceuticals have not admitted they were aware of the dangers of Dupixent, there is evidence that the companies knew or should have known.
Since 2017, the FDA has received over 365,000 adverse event reports related to Dupixent. While only a small percentage of these mention CTCL, there are enough complaints for the manufacturers to be aware of the potential crisis.
Dupixent is a medication that doctors and dermatologists prescribe to treat and manage atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, prurigo nodularis, and COPD.
Dupixent, the brand name for dupilumab, is a “monoclonal antibody” that inhibits interleukin-4 receptor alpha (IL-4Ra) and interleukin-13 receptor (IL-13R). These receptors play a role in inflammation from allergies.
How Dupixent works depends on the condition it treats. For example, Dupixent treats eczema by suppressing inflammation. It attaches to immune cells and inhibits the immune response, causing inflammation and atopic dermatitis. Dupixent treats asthma by blocking airway inflammation in the lungs that causes asthma.
CTCL can be treated. However, many patients who used Dupixent were not aware of the risk of lymphoma. This meant that many victims of Dupixent caught their cancer when it was untreatable. If you catch CTCL early enough, it may respond to treatment.
The side effects vary depending on the condition you are using Dupixent to treat, but they often include injection site reactions, eye problems such as itching, redness, inflammation, and blurred vision, cold sores, and a high count of a certain type of white blood cell.
While these are all potential side effects, you should still inform your doctor.
Dupixent can also cause allergic reactions, blood vessel inflammation, psoriasis, and joint pain.
As of September 2025, Dupixent does not have a Black Box warning. The FDA has not required any warnings or prompted any recalls for Dupixent, as of September 2025.
Receiving a diagnosis of cutaneous T-cell lymphoma CTCL after using Dupixent can feel overwhelming and raise serious questions about your health, treatment, and legal rights. Taking the right steps early can help protect both your well-being and your potential claim.
- Follow your doctor’s treatment plan. Your health comes first. Make sure you get the medical care you need and follow all recommended treatments.
- Keep thorough records. Save medical records, pathology reports, prescription information, and any communications from your healthcare providers. These documents may be crucial evidence later.
- Track your expenses and symptoms. Document medical bills, lost wages, and daily health impacts, including pain, fatigue, or emotional distress.
- Avoid stopping or changing medication without medical guidance. Only your treating doctor can advise you on safe next steps for your treatment.
- Contact our firm to connect with an experienced attorney in your area. We can review your situation, evaluate whether you may have a claim, explain your legal options, and guide you through the process.
- Don’t deal with the pharmaceutical company alone. Having a lawyer handle communications ensures you won’t be pressured into an unfair or premature settlement.
Get Help With Your Dupixent Lawsuit Today
If you or a loved one has suffered harmful side effects after using Dupixent, you don’t have to face the legal battle alone. Experienced lawyers who understand the complexities of pharmaceutical litigation can manage your case and hold the manufacturer accountable.
We can help connect you with one of our trusted law firm partners in your area who has the knowledge and resources to pursue your Dupixent lawsuit. Don’t wait—taking action today can help protect your rights and secure the compensation you need.
Contact The Goldwater Law Firm now to get started.