– Updated on May 22, 2025
Consult an Attorney to Determine if You’re Eligible to File an Allergan Textured Breast Implant Lawsuit
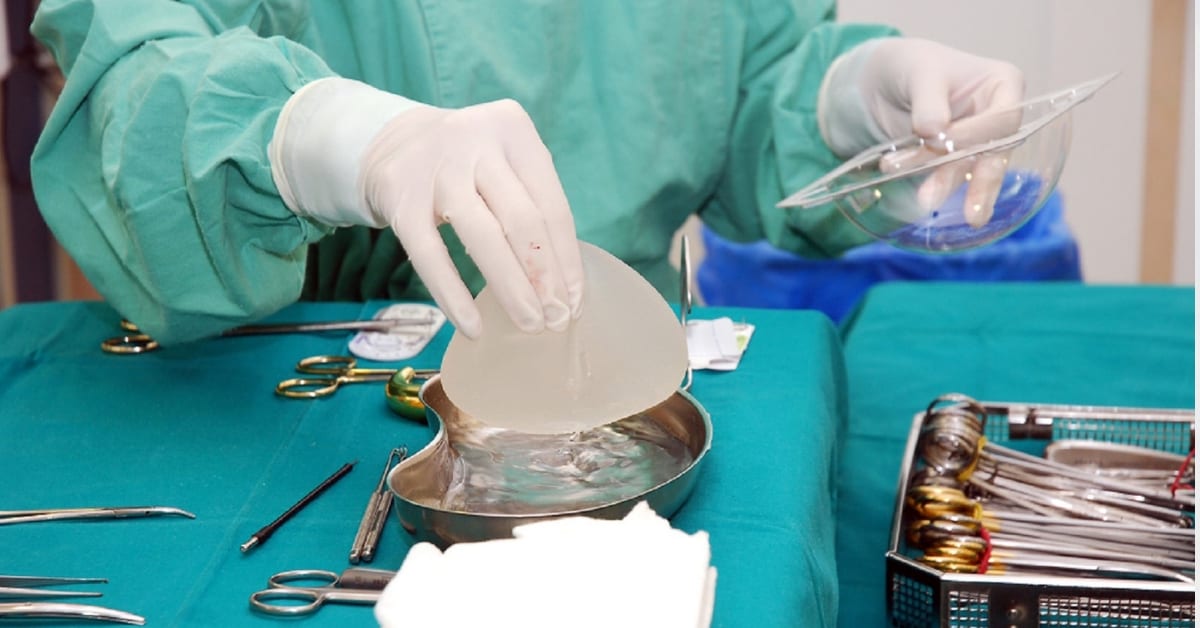
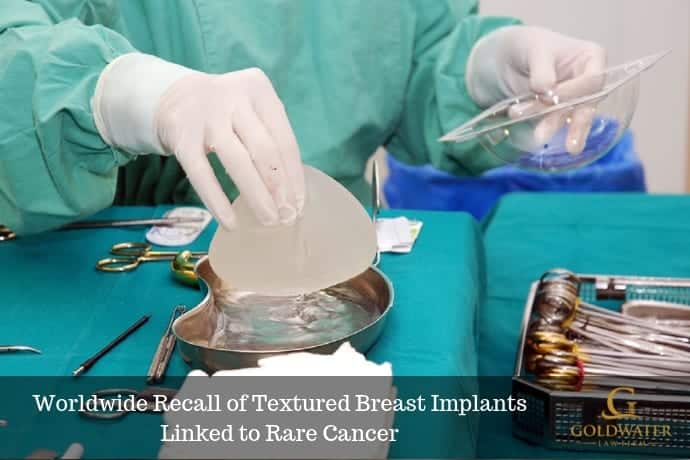
Allergan recently issued a worldwide recall of Biocell textured breast implants and tissue expanders. The decision was made after the US Food and Drug Administration (FDA) requested that the manufacturer voluntarily recall the products due to the risk of breast implant-associated anaplastic large cell lymphoma (BIA-ALCL).
It is estimated that hundreds of thousands of women have these implants, CNN reported. If you or a loved one has undergone breast implant surgery and has since been diagnosed with a breast-implant associated cancer, you may be able to file an Allergan textured breast implant lawsuit.
Here’s what you need to know about the recent recall and your legal rights.
Allergan Textured Breast Implants
In 2011, the FDA first identified a possible association between breast implants and the development of BIA-ALCL, a rare form of non-Hodgkin’s lymphoma.
“Although the overall incidence of BIA-ALCL appears to be relatively low, once the evidence indicated that a specific manufacturer’s product appeared to be directly linked to significant patient harm, including death, the FDA took action to alert the firm to new evidence indicating a recall is warranted to protect women’s health,” the FDA said in a news release.
The specific textured breast implant products that have been recalled include:
- Natrelle Saline-Filled breast implants
- Natrelle Silicone-Filled breast implants
- Natrelle Inspira Silicone-Filled breast implants
- Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled breast implants
The recalled tissue expanders include:
- Natrelle 133 Plus Tissue Expander
- Natrelle 133 Tissue Expander with Suture Tabs
The FDA has stated that they will continue to monitor and evaluate BIA-ALCL due to textured and smooth breast implants and tissue expanders, as well as any new information regarding other devices used for breast augmentation.
How Many Women Have Been Affected?
As of July 2019, 573 unique cases globally of anaplastic large cell lymphoma related to breast implants have been reported to the FDA. There have also been 33 patient deaths.
Specifically, of the 573 unique cases, 481 are attributed to Allergan implants, according to the FDA. Of the 33 patient deaths, 12 of the 13 patients for which the manufacturer of the implant is known, are confirmed to have an Allergan implant at the time of their diagnosis.
What Should Breast Implant Patients Do Now?
If you currently have textured breast implants and aren’t experiencing any symptoms of BIA-ALCL, the FDA doesn’t recommend removal, but rather that you understand the symptoms and continue to monitor the area around your implants. If you do notice any changes or experience any complications, it’s important that you consult your doctor immediately.
The symptoms associated with BIA-ALCL include persistent swelling or pain near the implant. Keep in mind that these symptoms can occur even years after the implant was placed.
The FDA is considering recommendations to change the labeling of breast implants to include a boxed warning indicating the health risks and other actions to share safety information, CNN reported.
If You’ve Been Diagnosed With Cancer
People who developed breast implant-caused cancer may require surgery, chemotherapy, and/or radiation therapy. If not treated quickly, BIA-ALCL can result in death.
If you’ve been diagnosed with breast implant-related cancer, you may be able to file a product injury claim against Allergan, Inc. to help you financially recover from the devastation caused by your BIA-ALCL diagnosis. You may be able to seek compensation for damages including but not limited to:
- Medical treatment
- Loss of wages
- Pain and suffering
Contact Goldwater Law Firm Today
Goldwater Law Firm is committed to holding negligent corporations responsible when their products cause physical and/or emotional damage to consumers. It can be incredibly challenging and intimidating to stand up to large corporations who have wronged you or a loved one.
At Goldwater Law Firm, we will put you in touch with one of our partner law firms that aren’t afraid to fight against these deep-pocketed corporations on your behalf. Don’t wait to take action. There may be time limits that affect your ability to obtain compensation. Contact us today at 866-984-9782, or fill out our form to find out if you’re able to file an Allergan textured breast implant lawsuit.

The Goldwater Law Firm is on mission to help as many people as possible with the fierce, compassionate legal aid only The Gold Standard of Injury Law can offer. If you suffered serious side effects or were diagnosed with an illness because of a defective drug or product, or if you were injured in an accident that wasn’t your fault, Attorney Bob Goldwater and the Goldwater Law Firm is ready to serve as your compassionate partner in the fight to seek the compensation and justice you deserve.
Share this post: